Oxidation of Carbon Compounds









Oxidation of Carbon Compounds
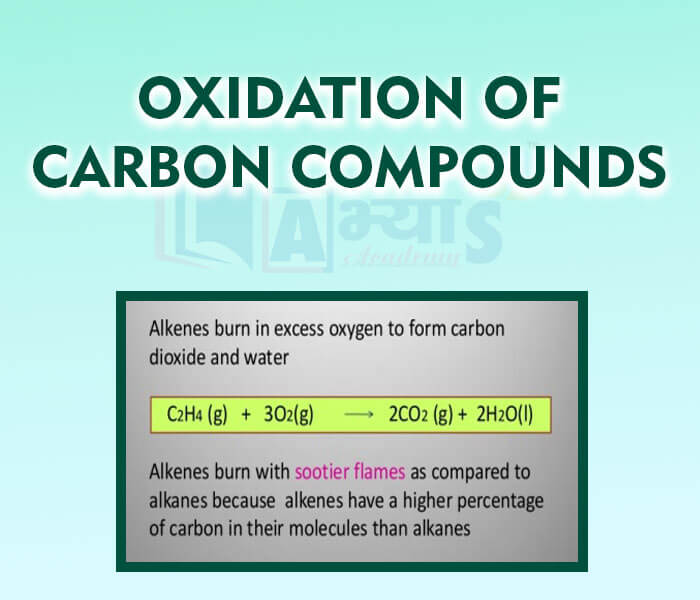
Oxidation:
It is the reaction involving the addition of oxygen or removal of hydrogen. Carbon undergo oxidation by combining with oxygen at higher temperature to form oxides. For eg: carbon monooxide,Carbon dioxide
etc. Carbon monoxide is formed by the incomplete combustion of carbon or carbon containing fuels.
Carbon dioxide may be prepared by complete combustion of carbon:
Assertion : Carbon monoxide is extremely poisonous in nature. Reason : Carbon monoxide is formed by complete combustion of carbon. | |||
| Right Option : C | |||
| View Explanation | |||
Which of the following are correct : (a) Alcohols can be prepared by heating carbon compounds in presence of oxidising agents like alkaline (b) Alcohols can be prepared by heating carbon compounds in presence of oxidising agents like acidified (c) Substances that are capable of adding oxygen to other substances are called oxidising agents. | |||
| Right Option : D | |||
| View Explanation | |||
Which of the following are correct : (a) Alcohols are converted into carboxylic acid only under complete oxidation. (b) In partial oxidation, alcohols are converted into aldehydes. (c) Oxidation is the reaction involving the addition of oxygen or removal of hydrogen. | |||
| Right Option : D | |||
| View Explanation | |||
Students / Parents Reviews [10]
One of the best institutes to develope a child interest in studies.Provides SST and English knowledge also unlike other institutes. Teachers are co operative and friendly online tests andPPT develope practical knowledge also.

Aman Kumar Shrivastava
10thIt has a great methodology. Students here can get analysis to their test quickly.We can learn easily through PPTs and the testing methods are good. We know that where we have to practice

Barkha Arora
10thAbhyas is a complete education Institute. Here extreme care is taken by teacher with the help of regular exam. Extra classes also conducted by the institute, if the student is weak.

Om Umang
10thBeing a parent, I saw my daughter improvement in her studies by seeing a good result in all day to day compititive exam TMO, NSO, IEO etc and as well as studies. I have got a fruitful result from my daughter.

Prisha Gupta
8thA marvelous experience with Abhyas. I am glad to share that my ward has achieved more than enough at the Ambala ABHYAS centre. Years have passed on and more and more he has gained. May the centre flourish and develop day by day by the grace of God.

Archit Segal
7thI have spent a wonderful time in Abhyas academy. It has made my reasoning more apt, English more stronger and Maths an interesting subject for me. It has given me a habbit of self studying

Yatharthi Sharma
10thMy experience with Abhyas is very good. I have learnt many things here like vedic maths and reasoning also. Teachers here first take our doubts and then there are assignments to verify our weak points.

Shivam Rana
7thAbhyas Methodology is very good. It is based on according to student and each child manages accordingly to its properly. Methodology has improved the abilities of students to shine them in future.

Manish Kumar
10thIt was a good experience with Abhyas Academy. I even faced problems in starting but slowly and steadily overcomed. Especially reasoning classes helped me a lot.

Cheshta
10thAbout Abhyas metholodology the teachers are very nice and hardworking toward students.The Centre Head Mrs Anu Sethi is also a brilliant teacher.Abhyas has taught me how to overcome problems and has always taken my doubts and suppoeted me.
